Disruption of Movement in Parkinson’s Disease
- academicmemories
- Jul 8, 2024
- 7 min read
By: Catherine Gooch
History
In his 1817 work titled “An Essay on the Shaking Palsy,” James Parkinson described a progressive condition in which individuals develop a tremor, typically in their hands or arms. At first, he says, it might seem just an inconvenience; however, as the disease progresses, individuals begin to have difficulty maintaining posture and find themselves falling forward while standing or walking. As the disease progresses further, individuals lose control of other muscles, including those involved in eating and speaking, and also experience dementia. This condition later became known as Parkinson’s Disease.
Background
Parkinson’s Disease is a neurodegenerative disorder characterized by many changes in movement, such as tremors, slow or stiff movement, and difficulty with balance. In addition to these motor symptoms, the disease is also associated with a variety of cognitive symptoms, including worsening memory and visuo-spatial abilities (Roheger et al. 2018). Although it has no known cause, the effects this condition has on motor control are a major area of research and will be the focus of this article.
Motor Control
Motor control, unsurprisingly, refers to the ways in which we control all the movements we make in our lives. Although movements like walking or reaching to grab something feel so effortless that we don’t need to put much conscious effort into it, they are actually very complex processes for our nervous system to coordinate. To walk to a particular place, for instance, the brain must first receive inputs about where you are now, where you want to go, and determine the best path to get there. Then, it sends signals to the relevant muscles to contract in such a way that they move the legs through the gait cycle. Importantly, both the magnitude and direction of the forces produced by each muscle vary throughout the duration of the cycle, adding another layer of complexity for the motor commands to account for. There are many hypotheses for how exactly these complex dynamic processes are carried out (Latash et al., 2010), but those will not be discussed here. The key takeaway is that many parts of both the central and peripheral nervous systems are involved in motor control, and damage to any of them can lead to difficulty in coordinating movement.
Basal Ganglia
The basal ganglia are a cluster of neurons located deep within the brain that play a major role in motor control. The basal ganglia have many components, including the striatum and substantia nigra. These two structures are connected via the nigrostriatal pathway, which contains neurons that release the neurotransmitter dopamine. Dopamine modulates the behavior of motor neurons throughout the brain and spinal cord, meaning that it is crucial for proper motor function (Vidal-Gadea & Pierce-Shimomura, 2012). In people with Parkinson’s Disease, however, the release of dopamine is impaired due to the loss of neurons in the substantia nigra and other areas (Davie, 2008). This diminished release of dopamine impairs the brain’s ability to control the activity of motor neurons, and is what causes the characteristic motor symptoms of Parkinson’s disease.
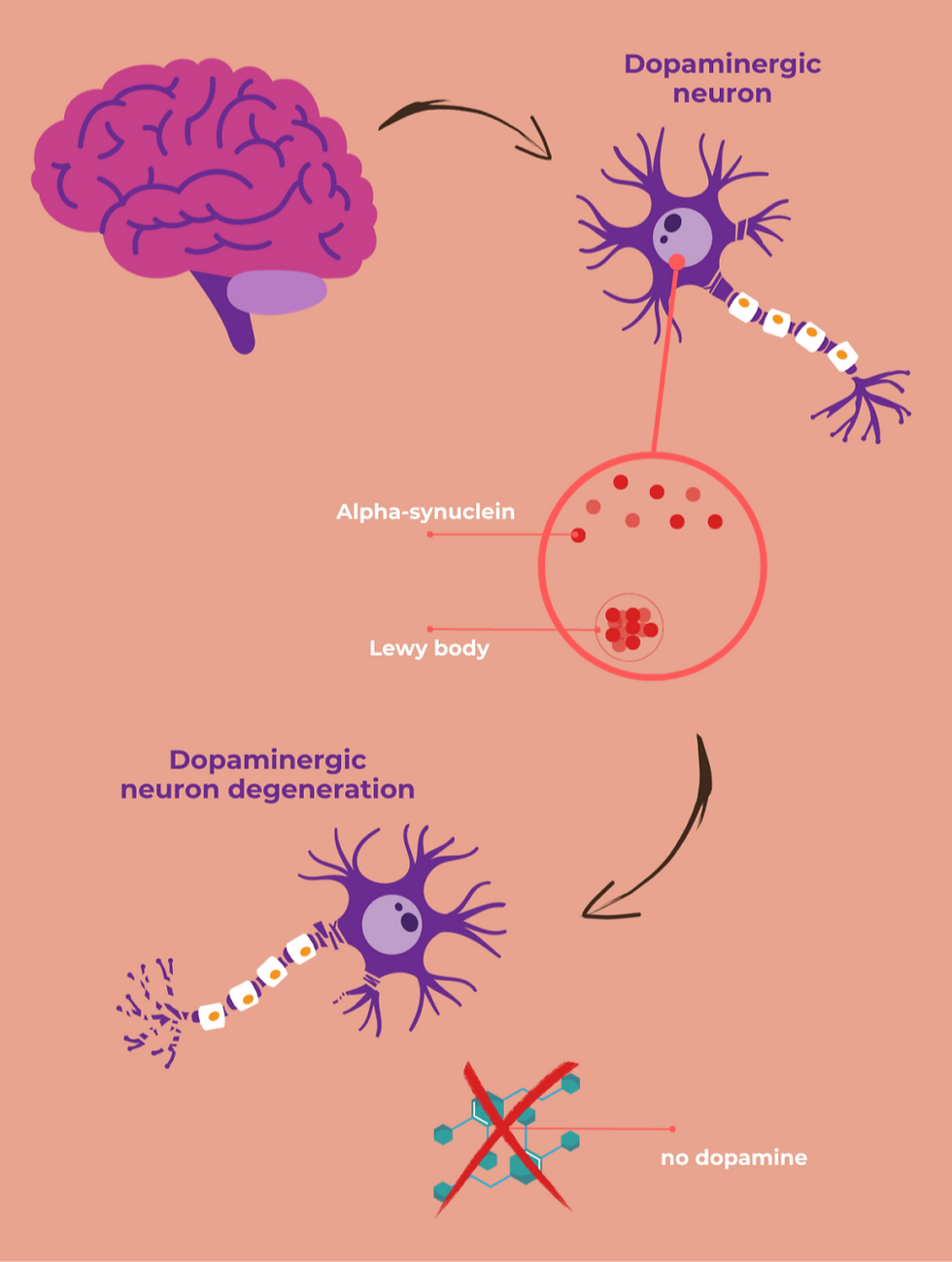
Lewy Bodies
Why do we see a loss of neurons in the basal ganglia? The answer lies with Lewy bodies, the key histological trait of Parkinson’s Disease. These are essentially abnormal clumps of a protein called alpha-synuclein. When these clumps appear inside neurons, they can disrupt the proper functioning of the cell and eventually lead to cell death.
In the early stages of Parkinson’s Disease, Lewy bodies begin to form in areas of the brain that lead to non-motor symptoms such as a loss of sense of smell (Burke et al., 2008). Eventually, they spread to other parts of the brain, notably the substantia nigra. This is when neurons in this area become affected and motor symptoms start to appear. As Parkinson's disease progresses, Lewy bodies continue to spread further, causing many other symptoms, including dementia.
Freezing of Gait
In addition to tremors and slow or rigid movement, another symptom that can occur in people with Parkinson’s Disease is freezing of gait. During these episodes, which typically last under a minute, individuals find themselves stuck, unable to move their feet off the ground no matter how much they try to walk. And although their feet are ‘frozen,’ the rest of the body continues to lean forward as if they are walking, often leading to falls. Although not everyone with Parkinson’s Disease experiences these episodes, they pose a major safety risk and are known to decrease quality of life (Walton et al., 2015) for those who do experience them.
Although the cause of freezing of gait is not fully understood, several models have been proposed for this phenomenon (Nieuwboer, 2013). For instance, some scientists suggest that freezing of gait occurs when deficits in multiple aspects of motor control add up and altogether cause a breakdown in the motor system, while others see it as being caused by multiple neural circuits being processed at the same time in the basal ganglia, essentially leading to wires getting crossed (Gao et al., 2020). Yet another model suggests that freezing of gait happens when there is a disconnect between the individual’s subconsciously anticipated movements and the actual movement pattern of stepping (Jacobs et al., 2009). While none of these models alone can fully explain every aspect of freezing of gait, they are useful for informing clinical treatment and finding avenues for further research.
Treatment
The main treatment for Parkinson’s Disease is a drug called levodopa, which helps to replace the missing dopamine in the brain. After crossing the blood-brain barrier, levodopa is converted to dopamine, allowing the nigrostriatal pathway and other areas to function more normally. It is typically taken in combination with carbidopa, which ensures that the rise in dopamine occurs inside the brain rather than in the periphery.
Management of Parkinson’s Disease can also be supplemented with certain types of physical therapy. One physical therapy program is known as amplitude training, which focuses on making big, overexaggerated movements to help maintain the ability to produce normally-sized movement. Additionally, some individuals benefit from gait training, where a physical therapist helps them to improve balance when walking. There are even robotic suits being developed, which provide support and reduce the energy required to walk (Kim et al. 2024).
Open Questions in Parkinson’s Research
Many aspects of Parkinson’s Disease are not yet fully understood. One type of question is about the extent to which certain symptoms are related to each other. For instance, people with the disease tend to perform individual movements slowly, but when asked to perform two movements either at the same time or one after the other, the total movement is slower than if you had just added the slower individual movements together (Schwab et al., 1954 ; Benecke et al., 1987). Here we have two very similar symptoms: difficulty with both individual and combined movements. But are these caused by the same underlying issue, or are they really two distinct symptoms? That is a difficult question to answer (Mazzoni et al., 2012).
Additionally, and perhaps more importantly, there is no known cause of Parkinson’s Disease. It is understood that the disease results from degeneration of the nigrostriatal pathway due to the presence of Lewy bodies, but we do not yet know why the alpha-synuclein proteins aggregate to form Lewy bodies in the first place. Certain gene variants have been implicated as risk factors for developing Parkinson’s Disease, but it is not considered genetic. Age is considered the largest risk factor as most people don’t show symptoms until their 60s, but there is currently no way to accurately predict who will develop the disease as they age and who won’t. This is an active area of research though, with scientists recently suggesting that a blood test might be able to identify the disease up to seven years prior to the first symptoms.
These questions and many others are being actively investigated by many scientists, and hopefully in the future there will be better technologies to both predict onset of Parkinson’s Disease before symptoms show, and to help individuals who are living with symptoms.
Sources:
Mazzoni, P., Shabbott, B., & Cortés, J. C. (2012). Motor control abnormalities in Parkinson's disease. Cold Spring Harbor perspectives in medicine, 2(6), a009282. https://doi.org/10.1101/cshperspect.a009282
Roheger, M., Kalbe, E., & Liepelt-Scarfone, I. (2018). Progression of Cognitive Decline in Parkinson's Disease. Journal of Parkinson's disease, 8(2), 183–193. https://doi.org/10.3233/JPD-181306
Opara, J., Małecki, A., Małecka, E., & Socha, T. (2017). Motor assessment in Parkinson`s disease. Annals of agricultural and environmental medicine : AAEM, 24(3), 411–415. https://doi.org/10.5604/12321966.1232774
Sauerbier, A., Qamar, M. A., Rajah, T., & Chaudhuri, K. R. (2016). New concepts in the pathogenesis and presentation of Parkinson's disease. Clinical medicine (London, England), 16(4), 365–370. https://doi.org/10.7861/clinmedicine.16-4-365
Vidal-Gadea, A. G., & Pierce-Shimomura, J. T. (2012). Conserved role of dopamine in the modulation of behavior. Communicative & integrative biology, 5(5), 440–447. https://doi.org/10.4161/cib.20978
Davie C. A. (2008). A review of Parkinson's disease. British medical bulletin, 86, 109–127. https://doi.org/10.1093/bmb/ldn013
Latash, M. L., Levin, M. F., Scholz, J. P., & Schöner, G. (2010). Motor control theories and their applications. Medicina (Kaunas, Lithuania), 46(6), 382–392.
Walton, C. C., Shine, J. M., Hall, J. M., O'Callaghan, C., Mowszowski, L., Gilat, M., Szeto, J. Y., Naismith, S. L., & Lewis, S. J. (2015). The major impact of freezing of gait on quality of life in Parkinson's disease. Journal of neurology, 262(1), 108–115. https://doi.org/10.1007/s00415-014-7524-3
Nieuwboer, A., & Giladi, N. (2013). Characterizing freezing of gait in Parkinson's disease: models of an episodic phenomenon. Movement disorders : official journal of the Movement Disorder Society, 28(11), 1509–1519. https://doi.org/10.1002/mds.25683
Gao, C., Liu, J., Tan, Y., & Chen, S. (2020). Freezing of gait in Parkinson's disease: pathophysiology, risk factors and treatments. Translational neurodegeneration, 9, 12. https://doi.org/10.1186/s40035-020-00191-5
Burke, R. E., Dauer, W. T., & Vonsattel, J. P. (2008). A critical evaluation of the Braak staging scheme for Parkinson's disease. Annals of neurology, 64(5), 485–491. https://doi.org/10.1002/ana.21541
Jacobs, J. V., Nutt, J. G., Carlson-Kuhta, P., Stephens, M., & Horak, F. B. (2009). Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Experimental neurology, 215(2), 334–341. https://doi.org/10.1016/j.expneurol.2008.10.019
Schwab, R. S., Chafetz, M. E., & Walker, S. (1954). Control of two simultaneous voluntary motor acts in normals and in parkinsonism. A.M.A. archives of neurology and psychiatry, 72(5), 591–598. https://doi.org/10.1001/archneurpsyc.1954.02330050061010
Benecke, R., Rothwell, J. C., Dick, J. P., Day, B. L., & Marsden, C. D. (1987). Disturbance of sequential movements in patients with Parkinson's disease. Brain : a journal of neurology, 110 ( Pt 2), 361–379. https://doi.org/10.1093/brain/110.2.361
Kim, J., Porciuncula, F., Yang, H. D., Wendel, N., Baker, T., Chin, A., Ellis, T. D., & Walsh, C. J. (2024). Soft robotic apparel to avert freezing of gait in Parkinson's disease. Nature medicine, 30(1), 177–185. https://doi.org/10.1038/s41591-023-02731-8
Hällqvist, J., Bartl, M., Dakna, M., Schade, S., Garagnani, P., Bacalini, M. G., Pirazzini, C., Bhatia, K., Schreglmann, S., Xylaki, M., Weber, S., Ernst, M., Muntean, M. L., Sixel-Döring, F., Franceschi, C., Doykov, I., Śpiewak, J., Vinette, H., Trenkwalder, C., Heywood, W. E., … Mollenhauer, B. (2024). Plasma proteomics identify biomarkers predicting Parkinson's disease up to 7 years before symptom onset. Nature communications, 15(1), 4759. https://doi.org/10.1038/s41467-024-48961-3

Comentarios